Integrin beta 3
| ITGB3 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | ITGB3, BDPLT16, BDPLT2, CD61, GP3A, GPIIIa, GT, integrin subunit beta 3, BDPLT24, GT2 | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 173470; MGI: 96612; HomoloGene: 55444; GeneCards: ITGB3; OMA:ITGB3 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Integrin beta-3 (β3) or CD61 is a protein that in humans is encoded by the ITGB3 gene.[5] CD61 is a cluster of differentiation found on thrombocytes.[6]
Structure and function
The ITGB3 protein product is the integrin beta chain beta 3. Integrins are integral cell-surface proteins composed of an alpha chain and a beta chain. A given chain may combine with multiple partners resulting in different integrins. Integrin beta 3 is found along with the alpha IIb chain in platelets. Integrins are known to participate in cell adhesion as well as cell-surface-mediated signaling.[7]
Role in endometriosis
Defectively expressed β3 integrin subunit has been correlated with presence of endometriosis, and has been suggested as a putative marker of this condition.[8]
Interactions
CD61 has been shown to interact with PTK2,[9][10] ITGB3BP,[11][12] TLN1[13][14] and CIB1.[15]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000259207 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000020689 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Sosnoski DM, Emanuel BS, Hawkins AL, van Tuinen P, Ledbetter DH, Nussbaum RL, Kaos FT, Schwartz E, Phillips D, Bennett JS, et al. (August 1988). "Chromosomal localization of the genes for the vitronectin and fibronectin receptors alpha subunits and for platelet glycoproteins IIb and IIIa". J Clin Invest. 81 (6): 1993–8. doi:10.1172/JCI113548. PMC 442653. PMID 2454952.
- ^ Heemskerk, J. W. M.; Mattheij, N. J. A.; Cosemans, J. M. E. M. (2013). "Platelet-based coagulation: different populations, different functions" (PDF). Journal of Thrombosis and Haemostasis. 11 (1): 2–16. doi:10.1111/jth.12045. ISSN 1538-7933. PMID 23106920. S2CID 206157070.
- ^ "Entrez Gene: ITGB3 integrin, beta 3 (platelet glycoprotein IIIa, antigen CD61)".
- ^ May, K. E.; Villar, J.; Kirtley, S.; Kennedy, S. H.; Becker, C. M. (2011). "Endometrial alterations in endometriosis: A systematic review of putative biomarkers". Human Reproduction Update. 17 (5): 637–653. doi:10.1093/humupd/dmr013. PMID 21672902.
- ^ Eliceiri, Brian P; Puente Xose S; Hood John D; Stupack Dwayne G; Schlaepfer David D; Huang Xiaozhu Z; Sheppard Dean; Cheresh David A (April 2002). "Src-mediated coupling of focal adhesion kinase to integrin alpha(v)beta5 in vascular endothelial growth factor signaling". J. Cell Biol. 157 (1): 149–60. doi:10.1083/jcb.200109079. ISSN 0021-9525. PMC 2173263. PMID 11927607.
- ^ Chung, J; Gao A G; Frazier W A (June 1997). "Thrombspondin acts via integrin-associated protein to activate the platelet integrin alphaIIbbeta3". J. Biol. Chem. 272 (23): 14740–6. doi:10.1074/jbc.272.23.14740. ISSN 0021-9258. PMID 9169439.
- ^ Fujimoto, Tetsuro-Takahiro; Katsutani Shinya; Shimomura Takeshi; Fujimura Kingo (January 2002). "Novel alternatively spliced form of beta(3)-endonexin". Thromb. Res. 105 (1): 63–70. doi:10.1016/S0049-3848(01)00405-4. ISSN 0049-3848. PMID 11864709.
- ^ Shattil, S J; O'Toole T; Eigenthaler M; Thon V; Williams M; Babior B M; Ginsberg M H (November 1995). "Beta 3-endonexin, a novel polypeptide that interacts specifically with the cytoplasmic tail of the integrin beta 3 subunit". J. Cell Biol. 131 (3): 807–16. doi:10.1083/jcb.131.3.807. ISSN 0021-9525. PMC 2120613. PMID 7593198.
- ^ Patil, S; Jedsadayanmata A; Wencel-Drake J D; Wang W; Knezevic I; Lam S C (October 1999). "Identification of a talin-binding site in the integrin beta(3) subunit distinct from the NPLY regulatory motif of post-ligand binding functions. The talin n-terminal head domain interacts with the membrane-proximal region of the beta(3) cytoplasmic tail". J. Biol. Chem. 274 (40): 28575–83. doi:10.1074/jbc.274.40.28575. ISSN 0021-9258. PMID 10497223.
- ^ Calderwood, David A; Yan Boxu; de Pereda Jose M; Alvarez Begoña García; Fujioka Yosuke; Liddington Robert C; Ginsberg Mark H (June 2002). "The phosphotyrosine binding-like domain of talin activates integrins". J. Biol. Chem. 277 (24): 21749–58. doi:10.1074/jbc.M111996200. ISSN 0021-9258. PMID 11932255.
- ^ Naik, U P; Patel P M; Parise L V (February 1997). "Identification of a novel calcium-binding protein that interacts with the integrin alphaIIb cytoplasmic domain". J. Biol. Chem. 272 (8): 4651–4. doi:10.1074/jbc.272.8.4651. ISSN 0021-9258. PMID 9030514.
Further reading
- Bray PF (1995). "Inherited diseases of platelet glycoproteins: considerations for rapid molecular characterization". Thromb. Haemost. 72 (4): 492–502. PMID 7878622.
- Bennett JS (2001). "Platelet-fibrinogen interactions". Ann. N. Y. Acad. Sci. 936 (1): 340–54. Bibcode:2001NYASA.936..340B. doi:10.1111/j.1749-6632.2001.tb03521.x. PMID 11460491. S2CID 25431334.
- Sajid M, Stouffer GA (2002). "The role of alpha(v)beta3 integrins in vascular healing". Thromb. Haemost. 87 (2): 187–93. doi:10.1055/s-0037-1612971. PMID 11858476.
- Quinn MJ, Byzova TV, Qin J, et al. (2004). "Integrin alphaIIbbeta3 and its antagonism". Arterioscler. Thromb. Vasc. Biol. 23 (6): 945–52. doi:10.1161/01.ATV.0000066686.46338.F1. PMID 12637342.
- Charakida M, Tousoulis D, Stefanadis C, Toutouzas P (2003). "The impact of platelet glycoprotein IIIa and Ia polymorphisms in cardiovascular thrombotic disease". Italian Heart Journal. 4 (1): 17–22. PMID 12690916.
- Smith FB, Connor JM, Lee AJ, et al. (2004). "Relationship of the platelet glycoprotein PlA and fibrinogen T/G+1689 polymorphisms with peripheral arterial disease and ischaemic heart disease". Thromb. Res. 112 (4): 209–16. doi:10.1016/j.thromres.2003.11.010. PMID 14987913.
- Fullard JF (2004). "The role of the platelet glycoprotein IIb/IIIa in thrombosis and haemostasis". Curr. Pharm. Des. 10 (14): 1567–76. doi:10.2174/1381612043384682. PMID 15134555.
- Cacciari B, Spalluto G (2005). "Non peptidic alphavbeta3 antagonists: recent developments". Curr. Med. Chem. 12 (1): 51–70. doi:10.2174/0929867053363522. PMID 15638730.
- Watson SP, Auger JM, McCarty OJ, Pearce AC (2005). "GPVI and integrin alphaIIb beta3 signaling in platelets". J. Thromb. Haemost. 3 (8): 1752–62. doi:10.1111/j.1538-7836.2005.01429.x. PMID 16102042. S2CID 42666713.
- Bennett JS (2006). "Structure and function of the platelet integrin alphaIIbbeta3". J. Clin. Invest. 115 (12): 3363–9. doi:10.1172/JCI26989. PMC 1297263. PMID 16322781.
External links
- CD61+Antigens at the U.S. National Library of Medicine Medical Subject Headings (MeSH)
- ITGB3 Info with links in the Cell Migration Gateway Archived 2014-12-11 at the Wayback Machine
- Human ITGB3 genome location and ITGB3 gene details page in the UCSC Genome Browser.
- v
- t
- e
-
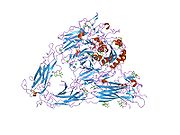 1jv2: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN ALPHAVBETA3
1jv2: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN ALPHAVBETA3 -
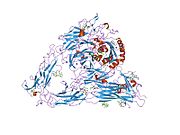 1l5g: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN AVB3 IN COMPLEX WITH AN ARG-GLY-ASP LIGAND
1l5g: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN AVB3 IN COMPLEX WITH AN ARG-GLY-ASP LIGAND -
 1m1x: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN ALPHA VBETA3 BOUND TO MN2+
1m1x: CRYSTAL STRUCTURE OF THE EXTRACELLULAR SEGMENT OF INTEGRIN ALPHA VBETA3 BOUND TO MN2+ -
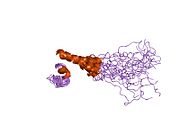 1m8o: Platelet integrin alfaIIb-beta3 cytoplasmic domain
1m8o: Platelet integrin alfaIIb-beta3 cytoplasmic domain -
 1txv:
1txv: -
 1ty3:
1ty3: -
 1ty5:
1ty5: -
 1ty6:
1ty6: -
 1ty7:
1ty7: -
 1tye: Structural basis for allostery in integrins and binding of ligand-mimetic therapeutics to the platelet receptor for fibrinogen
1tye: Structural basis for allostery in integrins and binding of ligand-mimetic therapeutics to the platelet receptor for fibrinogen -
 1u8c: A novel adaptation of the integrin PSI domain revealed from its crystal structure
1u8c: A novel adaptation of the integrin PSI domain revealed from its crystal structure
 | This article on a gene on human chromosome 17 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e






























