GDF5
| GDF5 | |||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Identifiers | |||||||||||||||||||||||||||||||||||||||||||||||||||
| Aliases | GDF5, BDA1C, BMP-14, BMP14, CDMP1, LAP-4, LAP4, OS5, SYM1B, SYNS2, growth differentiation factor 5, DUPANS | ||||||||||||||||||||||||||||||||||||||||||||||||||
| External IDs | OMIM: 601146; MGI: 95688; HomoloGene: 468; GeneCards: GDF5; OMA:GDF5 - orthologs | ||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
| Wikidata | |||||||||||||||||||||||||||||||||||||||||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||
Growth/differentiation factor 5 is a protein that in humans is encoded by the GDF5 gene.[5][6][7]
The protein encoded by this gene is closely related to the bone morphogenetic protein (BMP) family and is a member of the TGF-beta superfamily. This group of proteins is characterized by a polybasic proteolytic processing site which is cleaved to produce a mature protein containing seven conserved cysteine residues. The members of this family are regulators of cell growth and differentiation in both embryonic and adult tissues. Mutations in this gene are associated with acromesomelic dysplasia, Hunter-Thompson type; brachydactyly, type C; and osteochondrodysplasia, Grebe type. These associations confirm that the gene product plays a role in skeletal development.[7]
GDF5 is expressed in the developing central nervous system,[8] and has a role in skeletal and joint development.[9][10][11] It also increases the survival of neurones that respond to the neurotransmitter dopamine, and is a potential therapeutic molecule associated with Parkinson's disease.[12]
See also
References
- ^ a b c GRCh38: Ensembl release 89: ENSG00000125965 – Ensembl, May 2017
- ^ a b c GRCm38: Ensembl release 89: ENSMUSG00000038259 – Ensembl, May 2017
- ^ "Human PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ "Mouse PubMed Reference:". National Center for Biotechnology Information, U.S. National Library of Medicine.
- ^ Polinkovsky A, Robin NH, Thomas JT, Irons M, Lynn A, Goodman FR, Reardon W, Kant SG, Brunner HG, van der Burgt I, Chitayat D, McGaughran J, Donnai D, Luyten FP, Warman ML (Oct 1997). "Mutations in CDMP1 cause autosomal dominant brachydactyly type C". Nat Genet. 17 (1): 18–9. doi:10.1038/ng0997-18. hdl:2066/24464. PMID 9288091. S2CID 6580906.
- ^ Thomas JT, Kilpatrick MW, Lin K, Erlacher L, Lembessis P, Costa T, Tsipouras P, Luyten FP (Oct 1997). "Disruption of human limb morphogenesis by a dominant negative mutation in CDMP1". Nat Genet. 17 (1): 58–64. doi:10.1038/ng0997-58. PMID 9288098. S2CID 31479619.
- ^ a b "Entrez Gene: GDF5 growth differentiation factor 5 (cartilage-derived morphogenetic protein-1)".
- ^ O'Keeffe G, Dockery P, Sullivan A (2004). "Effects of growth/differentiation factor 5 on the survival and morphology of embryonic rat midbrain dopaminergic neurones in vitro". J Neurocytol. 33 (5): 479–88. doi:10.1007/s11068-004-0511-y. PMID 15906156. S2CID 25940876.
- ^ Buxton P, Edwards C, Archer C, Francis-West P (2001). "Growth/differentiation factor-5 (GDF-5) and skeletal development". J Bone Joint Surg Am. 83-A Suppl 1 (Pt 1): S23–30. PMID 11263662.
- ^ Francis-West P, Abdelfattah A, Chen P, Allen C, Parish J, Ladher R, Allen S, MacPherson S, Luyten F, Archer C (1999). "Mechanisms of GDF-5 action during skeletal development". Development. 126 (6): 1305–15. doi:10.1242/dev.126.6.1305. PMID 10021348.
- ^ Francis-West P, Parish J, Lee K, Archer C (1999). "BMP/GDF-signalling interactions during synovial joint development". Cell Tissue Res. 296 (1): 111–9. doi:10.1007/s004410051272. PMID 10199971. S2CID 21942870.
- ^ Sullivan A, O'Keeffe G (2005). "The role of growth/differentiation factor 5 (GDF5) in the induction and survival of midbrain dopaminergic neurones: relevance to Parkinson's disease treatment". J Anat. 207 (3): 219–26. doi:10.1111/j.1469-7580.2005.00447.x. PMC 1571542. PMID 16185246.
Further reading
- Reddi AH (1997). "Cartilage morphogenesis: role of bone and cartilage morphogenetic proteins, homeobox genes and extracellular matrix". Matrix Biol. 14 (8): 599–606. doi:10.1016/S0945-053X(05)80024-1. PMID 9057810.
- Luyten FP (1998). "Cartilage-derived morphogenetic protein-1". Int. J. Biochem. Cell Biol. 29 (11): 1241–4. doi:10.1016/S1357-2725(97)00025-3. PMID 9451821.
- Ducy P, Karsenty G (2000). "The family of bone morphogenetic proteins". Kidney Int. 57 (6): 2207–14. doi:10.1046/j.1523-1755.2000.00081.x. PMID 10844590.
- Faiyaz-Ul-Haque M, Ahmad W, Wahab A, et al. (2003). "Frameshift mutation in the cartilage-derived morphogenetic protein 1 (CDMP1) gene and severe acromesomelic chondrodysplasia resembling Grebe-type chondrodysplasia". American Journal of Medical Genetics. 111 (1): 31–7. doi:10.1002/ajmg.10501. PMID 12124730.
- Chang SC, Hoang B, Thomas JT, et al. (1994). "Cartilage-derived morphogenetic proteins. New members of the transforming growth factor-beta superfamily predominantly expressed in long bones during human embryonic development". J. Biol. Chem. 269 (45): 28227–34. doi:10.1016/S0021-9258(18)46918-9. PMID 7961761.
- Hötten G, Neidhardt H, Jacobowsky B, Pohl J (1994). "Cloning and expression of recombinant human growth/differentiation factor 5". Biochem. Biophys. Res. Commun. 204 (2): 646–52. doi:10.1006/bbrc.1994.2508. PMID 7980526.
- Thomas JT, Lin K, Nandedkar M, et al. (1996). "A human chondrodysplasia due to a mutation in a TGF-beta superfamily member". Nat. Genet. 12 (3): 315–7. doi:10.1038/ng0396-315. PMID 8589725. S2CID 43140478.
- Lin K, Thomas JT, McBride OW, Luyten FP (1996). "Assignment of a new TGF-beta superfamily member, human cartilage-derived morphogenetic protein-1, to chromosome 20q11.2". Genomics. 34 (1): 150–1. doi:10.1006/geno.1996.0257. PMID 8661040.
- Nishitoh H, Ichijo H, Kimura M, et al. (1996). "Identification of type I and type II serine/threonine kinase receptors for growth/differentiation factor-5". J. Biol. Chem. 271 (35): 21345–52. doi:10.1074/jbc.271.35.21345. PMID 8702914.
- Erlacher L, McCartney J, Piek E, et al. (1998). "Cartilage-derived morphogenetic proteins and osteogenic protein-1 differentially regulate osteogenesis". J. Bone Miner. Res. 13 (3): 383–92. doi:10.1359/jbmr.1998.13.3.383. PMID 9525338. S2CID 25307046.
- Sugiura T, Hötten G, Kawai S (1999). "Minimal promoter components of the human growth/differentiation factor-5 gene". Biochem. Biophys. Res. Commun. 263 (3): 707–13. doi:10.1006/bbrc.1999.1445. PMID 10512744.
- Aoki H, Fujii M, Imamura T, et al. (2001). "Synergistic effects of different bone morphogenetic protein type I receptors on alkaline phosphatase induction". J. Cell Sci. 114 (Pt 8): 1483–9. doi:10.1242/jcs.114.8.1483. PMID 11282024.
- Deloukas P, Matthews LH, Ashurst J, et al. (2002). "The DNA sequence and comparative analysis of human chromosome 20". Nature. 414 (6866): 865–71. Bibcode:2001Natur.414..865D. doi:10.1038/414865a. PMID 11780052.
- Faiyaz-Ul-Haque M, Ahmad W, Zaidi SH, et al. (2003). "Mutation in the cartilage-derived morphogenetic protein-1 (CDMP1) gene in a kindred affected with fibular hypoplasia and complex brachydactyly (DuPan syndrome)". Clin. Genet. 61 (6): 454–8. doi:10.1034/j.1399-0004.2002.610610.x. PMID 12121354. S2CID 28701236.
- v
- t
- e
-
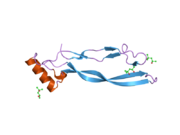 1waq: CRYSTAL STRUCTURE OF HUMAN GROWTH AND DIFFERENTIATION FACTOR 5 (GDF-5)
1waq: CRYSTAL STRUCTURE OF HUMAN GROWTH AND DIFFERENTIATION FACTOR 5 (GDF-5) -
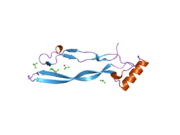 2bhk: CRYSTAL STRUCTURE OF HUMAN GROWTH AND DIFFERENTIATION FACTOR 5 (GDF5)
2bhk: CRYSTAL STRUCTURE OF HUMAN GROWTH AND DIFFERENTIATION FACTOR 5 (GDF5)
 | This article on a gene on human chromosome 20 is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e
 | This molecular or cell biology article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e




















