Selenite (ion)
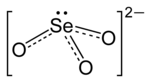
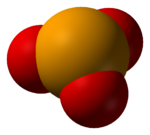

Selenite refers to the anion with the chemical formula SeO2−3. It is the oxyanion of selenium. It is the selenium analog of the sulfite ion, SO2−3. Thus selenite is pyramidal and selenium is assigned oxidation state +4. Selenite also refers to compounds that contains this ion, for example sodium selenite Na2SeO3 which is a common source of selenite.[1] Selenite also refers to the esters of selenous acid, for example dimethyl selenite (CH3)2SeO3.
Synthesis and reactions
Selenite salts can be prepared by neutralizing solutions of selenous acid, which is generated by dissolving selenium dioxide in water. The process proceeds via the hydrogenselenite ion, HSeO−3.
Selenite reacts with elemental sulfur to form thioselenate:[2]
- SeO2−3 + S → SSeO2−3
Most selenite salts can be formed by heating the metal oxide with selenium dioxide, e.g.:
- Na2O + SeO2 → Na2SeO3
References
- ^ F. Fehér (1963). "Sodium Selenite (IV)". In G. Brauer (ed.). Handbook of Preparative Inorganic Chemistry, 2nd Ed. Vol. 1pages=431. NY, NY: Academic Press.
- ^ Ball, Sheila; Milne, John (1995). "Studies on the Interaction of Selenite and Selenium with Sulfur Donors. Part 3. Sulfite". Canadian Journal of Chemistry. 73 (5): 716–724. doi:10.1139/v95-091.

- v
- t
- e
| H2SeO3 | He | ||||||||||||||||||||
| Li | Be | BxSexOx | C | +NO3 | O | +F | Ne | ||||||||||||||
| Na2SeO3 | Mg | Al | Si | P | +SO4 | Cl | Ar | ||||||||||||||
| K | Ca | Sc | Ti | V | Cr | MnSeO3 | Fe2(SeO3)3 | Co | Ni | CuSeO3 Cu2OSeO3 | ZnSeO3 | Ga | Ge | As | +SeO4 | Br | Kr | ||||
| RbHSeO3 | Sr | Y | Zr | Nb | Mo | Tc | Ru | Rh | Pd | Ag2SeO3 | Cd | In | Sn | Sb | Te | I | Xe | ||||
| CsHSeO3 | Ba | * | Lu | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg | Tl | Pb | Bi | Po | At | Rn | |||
| Fr | Ra | ** | Lr | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| * | La | Ce | Pr | Nd | Pm | Sm | Eu | Gd | Tb | Dy | Ho | Er | Tm | Yb | |||||||
| ** | Ac | Th | Pa | U | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | |||||||
 | This chemistry-related article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e











