Schwartz's reagent
 | |
 | |
| Names | |
|---|---|
| IUPAC name Chloridohydridozirconocene | |
| Systematic IUPAC name chloridobis(η5-cyclopentadienyl)hydridozirconium | |
| Other names Cp2ZrClH, zirconocene chloride hydride | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.048.599 |
| EC Number |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C10H11ClZr |
| Molar mass | 257.87 g/mol |
| Appearance | White solid |
| Hazards | |
| GHS labelling: | |
Pictograms |   |
| Danger | |
Hazard statements | H228, H261, H314 |
Precautionary statements | P210, P231+P232, P240, P241, P260, P264, P280, P301+P330+P331, P303+P361+P353, P304+P340, P305+P351+P338, P310, P321, P363, P370+P378, P402+P404, P405, P501 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  Y verify (what is Y verify (what is  Y Y N ?) N ?) Infobox references | |
Schwartz's reagent is the common name for the organozirconium compound with the formula (C5H5)2ZrHCl, sometimes called zirconocene hydrochloride or zirconocene chloride hydride, and is named after Jeffrey Schwartz, a chemistry professor at Princeton University. This metallocene is used in organic synthesis for various transformations of alkenes and alkynes.[1]
Preparation
The complex was first prepared by Wailes and Weigold.[2] It can be purchased or readily prepared by reduction of zirconocene dichloride with lithium aluminium hydride:
- (C5H5)2ZrCl2 + 1⁄4 LiAlH4 → (C5H5)2ZrHCl + 1⁄4 LiAlCl4
This reaction also affords (C5H5)2ZrH2, which is treated with methylene chloride to give Schwartz's reagent [3]
An alternative procedure that generated Schwartz's reagent from dihydride has also been reported.[4] Moreover, it's possible to perform an in situ preparation of (C5H5)2ZrHCl from zirconocene dichloride by using LiH. This method can also be used to synthesize isotope-labeled molecules, like olefines by employing Li2H or Li3H as reducing agents.[5]
Schwartz's reagent has a low solubility in common organic solvents.[6] The trifluoromethanesulfonate (C5H5)2ZrH(OTf) is soluble in THF.[7]
Structure
The complex adopts the usual "clam-shell" structure seen for other Cp2MXn complexes.[8] The dimetallic structure has been confirmed by Microcrystal electron diffraction.[9] The results are consistent with FT-IR spectroscopy, which established that the hydrides are bridging. Solid state NMR spectroscopy also indicates a dimeric structure. The X-ray crystallographic structure for the methyl compound (C5H5)4Zr2H2(CH3)2 compound is analogous.[10]
Uses in organic synthesis
Schwartz's reagent reduces amides to aldehydes.[11]
Vinylation of ketones in high yields is a possible use of Schwartz's reagent.[12]
Schwartz's reagent has been used in the synthesis of some macrolide antibiotics,[13][14] (−)-motuporin,[15] and antitumor agents.[16]
Hydrozirconation
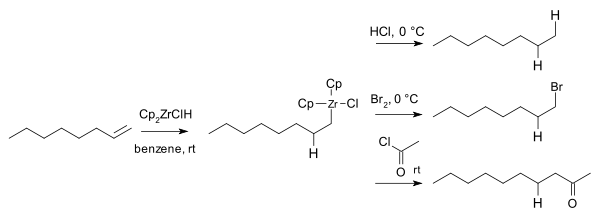
Hydrozirconation is a form of hydrometalation. Substrates for hydrozirconation are alkenes and alkynes. With terminal alkynes the terminal vinyl zirconium product is predominantly formed. Secondary reactions are nucleophilic additions, transmetalations,[17] conjugate additions,[18] coupling reactions, carbonylation and halogenation.
Computational studies indicate that hydrozirconation occurs from the interior portion.[19][20] When treated with one equivalent of Cp2ZrClH, diphenylacetylene gives the corresponding alkenylzirconium as a mixture of cis and trans isomers. With two equivalents of hydride, the endproduct was a mixture of erythro and threo zircono alkanes:
In 1974 Hart and Schwartz reported that the organozirconium intermediates react with electrophiles such as hydrochloric acid, bromine and acid chlorides to give the corresponding alkane, bromoalkanes, and ketones:[21]
The corresponding organoboron and organoaluminum compounds were already known, but these are air-sensitive and/or pyrophoric whereas organozirconium compounds are not.
Scope
In one study the usual regioselectivity of an alkyne hydrozirconation is reversed with the addition of zinc chloride:[22][23]
One example of a one-pot hydrozirconation - carbonylation - coupling is depicted below:[24][25]
With certain allyl alcohols, the alcohol group is replaced by nucleophilic carbon forming a cyclopropane ring:[26] The selectivity of the hydrozirconation of alkynes has been studied in detail.[27][28] Generally, the addition of the Zr–H proceeds via the syn-addition. The rate of addition to unsaturated carbon-carbon bonds is terminal alkyne > terminal alkene ≈ internal alkyne > disubstituted alkene [29] Acyl complexes can be generated by insertion of CO into the C–Zr bond resulting from hydrozirconation.[30] Upon alkene insertion into the zirconium hydride bond, the resulting zirconium alkyl undergoes facile rearrangement to the terminal alkyl and therefore only terminal acyl compounds can be synthesized in this way. The rearrangement most likely proceeds via β-hydride elimination followed by reinsertion.
Further reading
- Hart, D. W.; Schwartz, J. (1974). "Hydrozirconation. Organic Synthesis via Organozirconium Intermediates. Synthesis and Rearrangement of Alkylzirconium(IV) Complexes and Their Reaction with Electrophiles". J. Am. Chem. Soc. 96 (26): 8115–8116. doi:10.1021/ja00833a048.
- Schwartz, J.; Labinger, J. A. (2003). "Hydrozirconation: A New Transition Metal Reagent for Organic Synthesis". Angew. Chem. Int. Ed. 15 (6): 330–340. doi:10.1002/anie.197603331.
- Hart, Donald W.; Blackburn, Thomas F.; Schwartz, Jeffrey (1975). "Hydrozirconation. III. Stereospecific and regioselective functionalization of alkylacetylenes via vinylzirconium(IV) intermediates". J. Am. Chem. Soc. 97 (3): 679–680. doi:10.1021/ja00836a056.
References
- ^ Pinheiro, Danielle L. J.; De Castro, Pedro P.; Amarante, Giovanni W. (2018). "Recent Developments and Synthetic Applications of Nucleophilic Zirconocene Complexes from Schwartz's Reagent". European Journal of Organic Chemistry. 2018 (35): 4828–4844. doi:10.1002/ejoc.201800852. S2CID 102770378.
- ^ Wailes, P. C.; Weigold, H. (1970). "Hydrido complexes of zirconium I. Preparation". J. Organomet. Chem. 24 (2): 405–411. doi:10.1016/S0022-328X(00)80281-8.
- ^ Buchwald, Stephen L.; LaMaire, Susan J.; Nielsen, Ralph B.; Watson, Brett T.; King, Susan M. (1993). "Schwartz's Reagent". Org. Syntheses. 71: 77. doi:10.15227/orgsyn.071.0077.
- ^ Wipf, Peter; Takahashi, Hidenori; Zhuang, Nian (1998). "Kinetic vs. thermodynamic control in hydrozirconation reactions" (PDF). Pure Appl. Chem. 70 (5): 1077–1082. doi:10.1351/pac199870051077. S2CID 94092883.
- ^ Zippi, E. M.; Andres, H.; Morimoto, H.; Williams, P. G. (1994-04-01). "Preparation and Use of Tritiated Schwartz' Reagent (ZrCp2Cl3H)". Synthetic Communications. 24 (7): 1037–1044. doi:10.1080/00397919408020780. ISSN 0039-7911.
- ^ Wipf, Peter; Jahn, Heike (1996-09-30). "Synthetic applications of organochlorozirconocene complexes". Tetrahedron. 52 (40): 12853–12910. doi:10.1016/0040-4020(96)00754-5. ISSN 0040-4020.
- ^ Luinstra, Gerrit A.; Rief, Ursula; Prosenc, Marc H. (1995-04-01). "Synthesis and Reactivity of Cp2ZrH(OSO2CF3), a Soluble Monomeric Alternative for Schwartz's Reagent, and the Solid-State Structure of Its Dimer, [Cp2Zr(OSO2CF3)(-H)]2.0.5THF". Organometallics. 14 (4): 1551–1552. doi:10.1021/om00004a003. ISSN 0276-7333.
- ^ Wipf, Peter; Jahn, Heike (1996-09-30). "Synthetic applications of organochlorozirconocene complexes". Tetrahedron. 52 (40): 12853–12910. doi:10.1016/0040-4020(96)00754-5. ISSN 0040-4020.
- ^ Jones, Christopher G.; Asay, Matthew; Kim, Lee Joon; Kleinsasser, Jack F.; Saha, Ambarneil; Fulton, Tyler J.; Berkley, Kevin R.; Cascio, Duilio; Malyutin, Andrey G.; Conley, Matthew P.; Stoltz, Brian M.; Lavallo, Vincent; Rodríguez, José A.; Nelson, Hosea M. (6 September 2019). "Characterization of Reactive Organometallic Species via MicroED". ACS Central Science. 5 (9): 1507–1513. doi:10.1021/acscentsci.9b00403. PMC 6764211. PMID 31572777.
- ^ Rossini, A. J.; Mills, R. W.; Briscoe, G. A.; Norton, E. L.; Geier, S. J.; Hung, I.; Zheng, S.; Autschbach, J.; Schurko, R. W. (2009). "Solid-State Chlorine NMR of Group IV Transition Metal Organometallic Complexes". Journal of the American Chemical Society. 131 (9): 3317–3330. doi:10.1021/ja808390a. PMID 19256569.
- ^ Leighty, M. W.; Spletstoser, J. T.; Georg, Gunda I. (2011). "Mild Conversion of Tertiary Amides to Aldehydes Using Cp2ZrHCl (Schwartz's Reagent)". Org. Synth. 88: 427–437. doi:10.1002/0471264229.os088.39. ISBN 978-0471264224.
- ^ Li, H.; Walsh, P. J. (2005). "Catalytic Asymmetric Vinylation and Dienylation of Ketones". J. Am. Chem. Soc. 127 (23): 8355–8361. doi:10.1021/ja0425740. PMID 15941269.
- ^ Duffey, Matthew O.; Le Tiran, Arnaud; Morken, James P. (2003). "Enantioselective Total Synthesis of Borrelidin". J. Am. Chem. Soc. 125 (6): 1458–1459. doi:10.1021/ja028941u. PMID 12568588.
- ^ Wu, J.; Panek, J. S. (2011). "Total Synthesis of (−)-Virginiamycin M2: Application of Crotylsilanes Accessed by Enantioselective Rh(II) or Cu(I) Promoted Carbenoid Si–H Insertion". J. Org. Chem. 76 (24): 9900–9918. doi:10.1021/jo202119p. PMID 22070230.
- ^ Hu, T.; Panek, J. S. (1999). "Total Synthesis of (−)-Motuporin". J. Org. Chem. 64 (9): 3000–3001. doi:10.1021/jo9904617. PMID 11674393.
- ^ Nicolaou, K. C.; et al. (2003). "Total Synthesis of Apoptolidin: Completion of the Synthesis and Analogue Synthesis and Evaluation". J. Am. Chem. Soc. 125 (50): 15443–15454. doi:10.1021/ja030496v. PMID 14664590.
- ^ "Allylic alcohols by alkene transfer from zirconium to zinc: 1-[(tert-butyldiphenylsilyl)oxy]-dec-3-en-5-ol". Organic Syntheses. 9 (74): 205. 1998. Retrieved 2013-03-23.
Organic Syntheses, Coll. Vol. 9, p.143 (1998); Vol. 74, p.205 (1997).
- ^ Conjugate Addition Of A Vinylzirconium Reagent: 3-(1-Octen-1-Yl)Cyclopentanone, Organic Syntheses, Coll. Vol. 9, p.640 (1998); Vol. 71, p.83 (1993).
- ^ Pankratyev, E. Y.; Tyumkina, T. V.; Parfenova, L. V.; Khursan, S. L.; Khalilov, L. M.; Dzhemilev, U. M. (2011). "DFT and Ab Initio Study on Mechanism of Olefin Hydroalumination by XAlBui2 in the Presence of Cp2ZrCl2 Catalyst. II.(1) Olefin Interaction with Catalytically Active Centers". Organometallics. 30 (22): 6078–6089. doi:10.1021/om200518h.
- ^ Wang, Juping; Xu, Huiying; Gao, Hui; Su, Cheng-Yong; Zhao, Cunyuan; Phillips, David Lee (2010). "DFT Study on the Mechanism of Amides to Aldehydes Using Cp2Zr(H)Cl". Organometallics. 29 (1): 42–51. doi:10.1021/om900371u.
- ^ Hart, D. W.; Schwartz, J. (1974). "Hydrozirconation. Organic Synthesis via Organozirconium Intermediates. Synthesis and Rearrangement of Alkylzirconium(1V) Complexes and Their Reaction with Electrophiles". Journal of the American Chemical Society. 96 (26): 8115–8116. doi:10.1021/ja00833a048.
- ^ Zhang, Donghui (2007). "Directed Hydrozirconation of Propargylic Alcohols". Journal of the American Chemical Society. 129 (40): 12088–12089. doi:10.1021/ja075215o. PMC 2669288. PMID 17850152.
- ^ The electrophile in this reaction is iodine. The additive is believed to promote kinetic reaction control.
- ^ Kang, Suk-Ku (2002). "Palladium-catalyzed coupling reaction of acylzirconocene chlorides with hypervalent iodonium salts: synthesis of aryl-substituted ketones". Journal of the Chemical Society, Perkin Transactions 1 (4): 459–461. doi:10.1039/b110983a.
- ^ Reagents: phenylacetylene, Schwartz's reagent, tetraphenylpalladium and the iodane diphenyliodoniumtetrafluoroborate (phenyl group donor)
- ^ Gandon, Vincent (2002). "A one-pot access to cyclopropanes from allylic ethers via hydrozirconation–deoxygenative ring formation". Chemical Communications (12): 1308–1309. doi:10.1039/b203762a. PMID 12109129.
- ^ Sun, R. C.; Okabe, M.; Coffen, D. L.; Schwartz, J. (1998). "Conjugate Addition of a Vinylzirconium Reagent: 3-(1-Octene-1-yl)cyclopentanone". Organic Syntheses; Collected Volumes, vol. 9, p. 640.
- ^ Panek, J. S.; Hu, T. (1997). "Stereo- and Regiocontrolled Synthesis of Branched Trisubstituted Conjugated Dienes by Palladium(0)-Catalyzed Cross-Coupling Reaction". J. Org. Chem. 62 (15): 4912–4913. doi:10.1021/jo970647a.
- ^ Wipf, Peter; Jahn, Heike (1996). "Synthetic applications of organochlorozirconocene complexes". Tetrahedron. 52 (40): 12853–12910. doi:10.1016/0040-4020(96)00754-5.
- ^ Bertelo, Christopher A.; Schwartz, Jeffrey (1975). "Hydrozirconation. II. Oxidative homologation of olefins via carbon monoxide insertion into the carbon-zirconium bond". J. Am. Chem. Soc. 97 (1): 228–230. doi:10.1021/ja00834a061.
External links

- Examples in organic synthesis at the University of Connecticut website
- v
- t
- e
| CpH | He | ||||||||||||||||||||
| LiCp | Be | B | CpMe | N | C5H4O | F | Ne | ||||||||||||||
| NaCp | MgCp2 | Al | Si | P | S | Cl | Ar | ||||||||||||||
| K | CaCp2 | ScCp3 | TiCp2Cl2 (TiCp2Cl)2 | VCp2 VCpCh | CrCp2 (CrCp(CO)3)2 | MnCp2 | FeCp2 Fe(η5-C5H4Li)2 | CoCp2 CoCp(CO)2 | NiCp2 | Cu | Zn | Ga | Ge | As | Se | Br | Kr | ||||
| Rb | Sr | Y(C5H5)3 | ZrCp2Cl2 ZrCp2ClH | NbCp2Cl2 | MoCp2H2 MoCp2Cl2 | Tc | RuCp2 RuCp(PPh3)2Cl | RhCp2 | PdCp(C3H5) | Ag | Cd | InCp | SnCp2 | Sb | Te | I | Xe | ||||
| Cs | Ba | * | LuCp3 | HfCp2Cl2 | Ta | (WCp(CO)3)2 | ReCp2H | OsCp2 | IrCp2 | Pt | Au | Hg | TlCp | PbCp2 | Bi | Po | At | Rn | |||
| Fr | Ra | ** | Lr | Rf | Db | Sg | Bh | HsCp2 | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |||
| * | LaCp3 | CeCp3 | PrCp3 | NdCp3 | PmCp3 | SmCp3 | Eu | Gd | Tb | DyCp3 | Ho | ErCp3 | TmCp3 | YbCp3 | |||||||
| ** | Ac | ThCp3 ThCp4 | Pa | UCp4 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | |||||||