Scandium phosphide
 | |
| Names | |
|---|---|
| Other names Scandium monophosphide,[1] phosphanylidynescandium | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.032.153 |
| EC Number |
|
PubChem CID |
|
InChI
| |
| |
| Properties | |
Chemical formula | ScP |
| Molar mass | 75.929670 g·mol−1 |
| Structure[2] | |
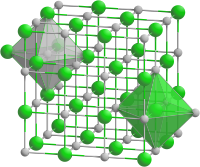
Crystal structure | Rock salt structure |
Space group | Fm3m |
Lattice constant | a = 0.5312 nm |
Formula units (Z) | 4 |
Coordination geometry | Octahedral at Sc3+, Octahedral at P3- |
| Related compounds | |
Other anions |
|
Other cations |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Scandium phosphide is an inorganic compound of scandium and phosphorus with the chemical formula ScP.[3][4][5]
Synthesis
ScP can be obtained by the reaction of scandium and phosphorus at 1000 °C.[2]
- 4 Sc + P4 → 4 ScP
Physical properties
This compound is calculated to be a semiconductor used in high power, high frequency applications and in laser diodes.[6][7]
Chemical properties
ScP can be smelted with cobalt or nickel through electric arc to obtain ScCoP and ScNiP.[8]
References
- ^ Gschneidner (Jr.), Karl A.; Eyring, LeRoy (1978). Handbook on the Physics and Chemistry of Rare Earths: without special title. North-Holland Publishing Company. p. 287. ISBN 978-0-444-82507-0. Retrieved 10 December 2021.
- ^ a b Parthé, E. (10 January 1963). "Note on the structure of ScP and YP". Acta Crystallographica. 16 (1): 71. Bibcode:1963AcCry..16...71P. doi:10.1107/S0365110X63000141. Retrieved 12 December 2021.
- ^ "Scandium Phosphide". American Elements. Retrieved 10 December 2021.
- ^ "scandium phosphide". National Institute of Standards and Technology. Retrieved 10 December 2021.
- ^ Toxic Substances Control Act (TSCA) Chemical Substance Inventory. U.S. Government Printing Office. 1979. p. 79. Retrieved 10 December 2021.
- ^ Karil, Poornima; Karma, Nikita; Choudhary, K. K.; Kaurav, Netram (29 May 2020). "Effect of pressure on structural and elastic properties of Scandium phosphide". AIP Conference Proceedings. Emerging Interfaces of Physical Sciences and Technology 2019: Eipt2019. 2224 (1): 030001. Bibcode:2020AIPC.2224c0001K. doi:10.1063/5.0000475. S2CID 219883570. Retrieved 10 December 2021.
- ^ Perkins, Peter G.; Marwaha, Ashok K.; Stewart, James J. P. (1 November 1981). "The band structures and magnetic properties of some transition-metal monophosphides I. Scandium phosphide". Theoretica Chimica Acta. 59 (6): 555–568. doi:10.1007/BF00552849. S2CID 94901262. Retrieved 10 December 2021.
- ^ Kleinke, Holger; Franzen, Hugo F. (1 May 1998). "Sc–Sc Bonding in the New Ternary Phosphide ScNiP". Journal of Solid State Chemistry. 137 (2): 218–222. Bibcode:1998JSSCh.137..218K. doi:10.1006/jssc.1997.7704. Retrieved 12 December 2021.
- v
- t
- e











