Pindone
 | |
| Names | |
|---|---|
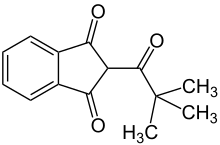
| Preferred IUPAC name 2-(2,2-Dimethylpropanoyl)-1H-indene-1,3(2H)-dione | |
| Other names 2-Pivaloyl-1,3-indandione | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChemSpider |
|
| ECHA InfoCard | 100.001.330 |
| KEGG |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C14H14O3 |
| Molar mass | 230.26 g/mol |
| Appearance | Bright-yellow powder[1] |
| Odor | almost none |
| Density | 1.06 g/mL |
| Melting point | 110 °C (230 °F; 383 K) |
Solubility in water | 0.002% (25°C)[1] |
| Hazards | |
| Lethal dose or concentration (LD, LC): | |
LD50 (median dose) | 280 mg/kg (rat, oral) 75 mg/kg (dog, oral) 150 mg/kg (rabbit, oral)[2] |
| NIOSH (US health exposure limits): | |
PEL (Permissible) | TWA 0.1 mg/m3[1] |
REL (Recommended) | TWA 0.1 mg/m3[1] |
IDLH (Immediate danger) | 100 mg/m3[1] |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa).  N verify (what is N verify (what is  Y Y N ?) N ?) Infobox references | |
Chemical compound
Pindone is an anticoagulant drug[3] for agricultural use. It is commonly used as a rodenticide in the management of rat and rabbit populations.
It is pharmacologically analogous to warfarin and inhibits the synthesis of Vitamin K-dependent clotting factors.
See also
References
- ^ a b c d e NIOSH Pocket Guide to Chemical Hazards. "#0516". National Institute for Occupational Safety and Health (NIOSH).
- ^ "Pindone". Immediately Dangerous to Life or Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH).
- ^ Robinson MH, Twigg LE, Wheeler SH, Martin GR (March 2005). "Effect of the anticoagulant, pindone, on the breeding performance and survival of merino sheep, Ovis aries". Comp. Biochem. Physiol. B. 140 (3): 465–73. doi:10.1016/j.cbpc.2004.11.011. PMID 15694595.
- v
- t
- e
Vitamin K antagonists
| Coumarins / 4-Hydroxycoumarins |
| ||||
|---|---|---|---|---|---|
| 1,3-Indandiones |
|
- Aluminium phosphide
- Arsenic
- Barium carbonate
- Calcium phosphide
- Cyanide
- Thallium sulfate
- Zinc phosphide
 | This agriculture article is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e
 | This article about a ketone is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e










