Glabrene
 | |
| Names | |
|---|---|
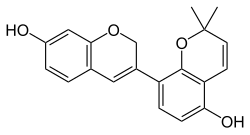
| IUPAC name 6′′,6′′-Dimethyl-6′′H-pyrano[2′′,3′′:2′,3′]isoflav-3-ene-4′,7-diol | |
| Systematic IUPAC name 2′,2′-Dimethyl-2H,2′H-[3,8′-bi-1-benzopyran]-5′,7-diol | |
| Other names 2',2'-dimethyl-2H,2'H-3,8'-bichromene-5',7-diol | |
| Identifiers | |
CAS Number |
|
3D model (JSmol) |
|
| ChEMBL |
|
| ChemSpider |
|
PubChem CID |
|
| UNII |
|
CompTox Dashboard (EPA) |
|
InChI
| |
| |
| Properties | |
Chemical formula | C20H18O4 |
| Molar mass | 322.36 g/mol |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). Infobox references | |
Chemical compound
Glabrene is an isoflavonoid that is found in Glycyrrhiza glabra (licorice).[1] It has estrogenic activity, showing estrogenic effects on breast, vascular, and bone tissue, and hence is a phytoestrogen (IC50 for estrogen receptor binding = 1 μM).[1][2][3] It has also been found to act as a tyrosinase inhibitor (IC50 = 3.5 μM) and to inhibit the formation of melanin in melanocytes, and for these reasons, has been suggested as a potential skin-lightening agent.[4]
See also
References
- ^ a b Tamir S, Eizenberg M, Somjen D, Izrael S, Vaya J (2001). "Estrogen-like activity of glabrene and other constituents isolated from licorice root". J. Steroid Biochem. Mol. Biol. 78 (3): 291–8. doi:10.1016/s0960-0760(01)00093-0. PMID 11595510. S2CID 40171833.
- ^ Somjen D, Knoll E, Vaya J, Stern N, Tamir S (2004). "Estrogen-like activity of licorice root constituents: glabridin and glabrene, in vascular tissues in vitro and in vivo". J. Steroid Biochem. Mol. Biol. 91 (3): 147–55. doi:10.1016/j.jsbmb.2004.04.003. PMID 15276622. S2CID 41966251.
- ^ Somjen D, Katzburg S, Vaya J, Kaye AM, Hendel D, Posner GH, Tamir S (2004). "Estrogenic activity of glabridin and glabrene from licorice roots on human osteoblasts and prepubertal rat skeletal tissues". J. Steroid Biochem. Mol. Biol. 91 (4–5): 241–6. doi:10.1016/j.jsbmb.2004.04.008. PMID 15336701. S2CID 16238533.
- ^ Nerya O, Vaya J, Musa R, Izrael S, Ben-Arie R, Tamir S (2003). "Glabrene and isoliquiritigenin as tyrosinase inhibitors from licorice roots". J. Agric. Food Chem. 51 (5): 1201–7. doi:10.1021/jf020935u. PMID 12590456.
- v
- t
- e
| Flavanones | |
|---|---|
| Flavones | |
| Prenylflavonoids |
|
| Isoflavones | |
| Isoflavanes | |
| Dihydrochalcones | |
| Isoflavenes | Glabrene |
| Coumestans |
|
| Lignans | |
| Flavonolignans | |
| Flavonols | |
| Others |
|
| Derivatives |
|
|---|
 | This article about an organic compound is a stub. You can help Wikipedia by expanding it. |
- v
- t
- e












